✔ 100% Authentic Product
👁️ Currently Viewing 641
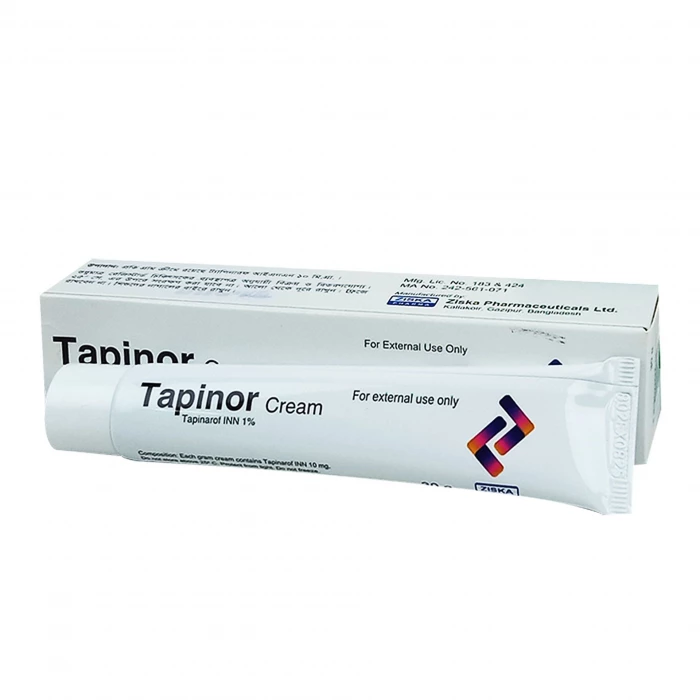
Pinarof contains Tapinarof as the active ingredient. Tapinarof is an Aryl Hydrocarbon Receptor (AhR) agonist. The specific mechanisms by which Tapinarof cream exerts its therapeutic action in psoriasis patients are unknown.
Discount
Price: ৳ 1,425
MRP:
৳
1500
5%
Off

100% Genuine Products, Guaranteed

Safe & Secure Payments, Always

Fast, Secure & Efficient Delivery

Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
🌙 রমযান অফার 🌙
 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
-
Google Play Store থেকে ডাউনলোড
-
Apple Store থেকে ডাউনলোড
100% Genuine Products, Guaranteed
Safe & Secure Payments, Always
Fast, Secure & Efficient Delivery
Proper Packaging
 Cash on Delivery - All over Bangladesh
Cash on Delivery - All over Bangladesh Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59
Regular Delivery - 12-24 Hours, Dhaka City* Charge Tk.39-59 Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110
Regular Delivery - 24-48 Hours, Other Cities* Charge Tk.99-110 ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে
ফ্রি ডেলিভারিঃ - ৭৯৯ টাকা+ অর্ডারে, ঢাকা
শহরে ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে
ফ্রি ডেলিভারিঃ - ২৭৯৯ টাকা+ অর্ডারে, ঢাকার
বাহিরে- Google Play Store থেকে ডাউনলোড
- Apple Store থেকে ডাউনলোড
🌙 রমযান অফার 🌙
📲 মোবাইল অ্যাপ অর্ডারে সাশ্রয় বেশী
✅ Description:
Pinarof 1% cream is indicated for the topical treatment of plaque psoriasis in adults. Apply a thin layer of Tapinarof cream to affected areas once daily. Wash hands after application, unless Tapisis cream is for the treatment of the hands. Tapinarof cream is not for oral, ophthalmic, or intravaginal use.
The safety and efficacy of Tapinarof cream have not been established in pediatric subjects with psoriasis under 18 years of age.
✔️ Side Effects
Most common adverse reactions (incidence ≥ 1%) in subjects treated with Tapisis cream were folliculitis, nasopharyngitis, contact dermatitis, headache, pruritus, and influenza.
✔️ Interaction
No hazardous interactions have been reported with other medicines.
✔️ Contraindications
Hypersensitive to any of its components.
✔️ Pregnancy & Lactation
Pregnancy
There are insufficient data on the use of Tapinarof Cream in pregnant women to determine any drug-associated risk of major birth defects, miscarriage, or adverse maternal or fetal outcomes.
In animal studies, subcutaneous administration of Tapinarof to pregnant rats and rabbits during organ development did not cause harmful effects at exposure levels approximately 268 times (rats) and 16 times (rabbits) higher than the maximum recommended human dose (MRHD).
The baseline risk of major birth defects or miscarriage in the treated population is unknown. However, all pregnancies carry a background risk of fetal abnormalities, loss, or other adverse outcomes.
Lactation
Information regarding the presence of Tapinarof in human breast milk, its effects on breastfed infants, or its impact on milk production is currently unavailable.
Tapinarof has been detected in the offspring of lactating rats after subcutaneous administration to the mother, indicating that the drug passes into animal milk. Therefore, it is likely that Tapinarof may be present in human milk as well.
When prescribing Tapinarof Cream to breastfeeding women, clinicians should weigh the benefits of breastfeeding against the mother’s clinical need for treatment and the potential risks to the nursing infant from either Tapinarof exposure or the underlying maternal condition.
✔️ Pregnancy & Lactation
For external use only. Avoid use in the eyes, oral or intravaginal use. If there is any unusual allergic reaction with the use of Pinarof, then treatment should be discontinued and appropriate therapy should be instituted.
✔️ Storage:
Do not store above 30°C. Keep in a dry place. Protect from light and keep out of the reach of children.
⚠️Disclaimer:
At ePharma, we’re committed to providing accurate and accessible health information. However, all content is intended for informational purposes only and should not replace medical advice from a qualified physician. Please consult your healthcare provider for personalized guidance. We aim to support, not substitute, the doctor-patient relationship.